As fuel cell vehicles rollout in California, Germany, Japan, the UK and elsewhere, creating an abundance of hydrogen is key to the success of the adoption of the vehicles. As fuel cell vehicles are considered to be disruptive technology so are the means of making hydrogen gas. One of the new ways of doing so is to use microwaves to create hydrogen from fossil fuels. The left over high-purity carbon can be sold in the marketplace for a whole host of applications such as carbon nanotubes for fuel cells.
Scientists have proven that microwaves can aid in creating nanostructured molybdenum disulphide or MoS2 catalysts with an enhancement that allows the production of hydrogen. The microwave-assisted approach functions by expanding the space and, therefore, diminishing the interaction between particular layers of MoS2 nanosheets. As a consequence, a larger portion of reactive sites end up being exposed, while on their edges hydrogen can be produced.
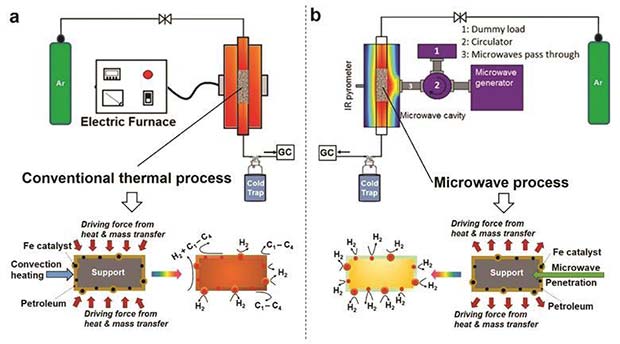
Scientists from both United Kingdom and Saudi Arabia established a technique that allows the production of high purity hydrogen from fossil fuels, without the natural release of carbon dioxide into the atmosphere. The process called microwave-initiated dehydrogenation functions based on inexpensive iron particle catalysts, while transforming unrefined petroleum into diesel, petrol or methane. Even though some view this as an innovative technique that should be put into practice in order to lessen the burden from our planet, a couple of specialists in the field criticise the approach and remain skeptical about its efficiency.
Furthermore, several experiments conducted at the University of Oxford by Pete Edwards and his team revealed more details in regards to microwaves creating hydrogen from fossil fuels. When it comes to the employment of hydrogen as a clean energy conductor, there are a few limitations that arise due to its lack of infrastructure, safety concerns and even the serviceable sources. Hence, researchers from Oxford aimed to cultivate a competent route that can dispose hydrogen at a higher rate from naturally prevailed fossil fuels, while taking advantage of the available infrastructure, due to the fact that those sources will still be used in the nearby future.
The conclusion that was drawn by Pete Edwards and his team was that the concept they discovered and used in their experiments reveals the fact that two scientific domains can work together harmoniously. Moreover, combining fundamental physics and chemical catalysis allowed them to observe how efficient can be to make use of induced metal-to-insulator transitions that take place when metal particles diminish to the point where quantum effects take place.
In other words, the microwave absorption rises to a fairly high level only when the electronic conductivity available in a particle diminishes drastically. By testing this theory on inexpensive iron particles, Edward`s team was able to successfully create microwave-absorbing catalytic particles that led to the creation of fuels like oil or methane.
Researchers at the University of Oxford state that their goal and vision remains to make use of hydrocarbon fuels through available distribution systems that permit a smooth supply of clean hydrogen, without the disposal of carbon dioxide emissions. The by-product of this process, the solid carbon, is a great source of catalysts that can be preserved underground or sold at a premium in the marketplace as I had previously mentioned.
As earth’s climate deteriorates rapidly, the means for producing hydrogen cleanly needs to increase just as rapidly. Microwaves may just be one answer to the equation.
Citation