In a significant leap forward for renewable energy technology, a research team led by materials scientists and chemists at UCLA has developed an innovative ultrafine platinum nanocatalyst embedded with cobalt oxide clusters. This groundbreaking advancement promises to make fuel cells more durable, nearly doubling the projected lifetime target set by the U.S. Department of Energy (DOE). The findings, recently published in Nature Catalysis, highlight the potential of this new catalyst to revolutionize the efficiency and longevity of fuel cells, offering a promising path toward more sustainable energy solutions.
The Challenge of Fuel Cell Durability
Fuel cells, particularly proton-exchange membrane (PEM) fuel cells, are a cornerstone of zero-emission power generation technology. They convert the chemical energy in hydrogen directly into electricity, with water vapor as the only emission byproduct. This makes them an attractive option for reducing carbon footprints and combating climate change. However, a significant challenge in the widespread adoption of fuel cells has been their durability. Traditional platinum-based catalysts, while effective in facilitating the necessary chemical reactions, tend to degrade over time, leading to reduced performance and the need for frequent replacements.
The Innovation: Ultrafine Platinum Nanocatalysts with Cobalt Oxide Clusters
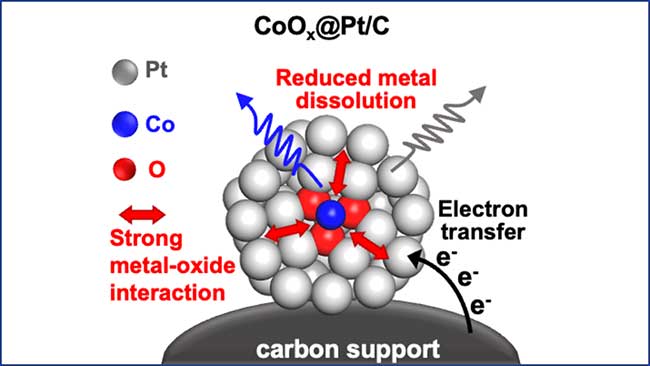
The UCLA research team, led by Professor Yu Huang from the Samueli School of Engineering, has addressed this challenge by designing an ultrafine platinum nanocatalyst with embedded cobalt oxide clusters. This novel approach leverages the strong interaction between platinum and cobalt oxide to enhance the structural and chemical stability of the catalyst.
Key Features of the New Catalyst:
Enhanced Durability: The embedded cobalt oxide clusters act as a scaffold, holding the platinum atoms in place and preventing them from dissolving over time. This significantly increases the catalyst’s durability, allowing it to maintain high performance over extended periods.
Increased Efficiency: By optimizing the interaction between platinum and cobalt oxide, the researchers have achieved a catalyst that not only lasts longer but also operates more efficiently. This means that fuel cells equipped with this new catalyst can generate more electricity from the same amount of hydrogen.
Reduced Costs: The improved durability and efficiency of the catalyst reduce the frequency of replacements, lowering the overall cost of fuel cell operation. This makes fuel cell technology more economically viable for a broader range of applications.
Implications for Light-Duty and Heavy-Duty Vehicles
The potential applications of this new catalyst are vast, particularly in the transportation sector. The researchers estimate that light-duty vehicles, such as passenger cars, equipped with the new durable fuel cells could last beyond 15,000 hours of use—87.5% longer than the DOE’s ultimate goal of 8,000 hours, or roughly 150,000 miles. This extended lifespan makes fuel cell vehicles a more attractive option for consumers, reducing the need for frequent maintenance and replacements.
For heavy-duty vehicles, such as long-haul semi-trucks, the benefits are equally significant. The increased durability and efficiency of the new catalyst can help these vehicles operate more reliably and cost-effectively, supporting the transition to cleaner transportation options.
The Science Behind the Breakthrough
The success of the new catalyst lies in its unique structure. Rather than using a traditional platinum alloy, the researchers embedded clusters of cobalt-oxide molecules inside shells of platinum atoms. This design leverages the strong platinum-oxide interaction, which makes the catalyst more durable structurally and chemically without sacrificing fuel cell activity.
In their experiments, the researchers demonstrated that this hybrid structure helps the platinum ions stick together despite extended use, reducing the rate of platinum dissolution. This not only enhances the catalyst’s longevity but also maintains its high catalytic activity over time.
Verification and Future Prospects
The team verified the nanoscale structure and performance of the new catalyst using a suite of advanced microscopic, spectroscopic, and simulation techniques. These comprehensive analyses confirmed that the new catalyst outperformed traditional platinum-cobalt alloys in both durability and efficiency.
Looking ahead, the researchers are optimistic about the potential of their innovation to transform the fuel cell industry. They believe that further refinements and scaling up of the production process could lead to even greater improvements in performance and cost-effectiveness.
Conclusion
The development of the ultrafine platinum nanocatalyst with embedded cobalt oxide clusters by UCLA’s research team represents a major advancement in fuel cell technology. By nearly doubling the projected lifetime target set by the DOE, this breakthrough addresses one of the key challenges in the adoption of fuel cells: durability. The enhanced efficiency and reduced costs associated with the new catalyst make it a promising solution for both light-duty and heavy-duty vehicles, supporting the broader transition to sustainable energy.
As the world continues to seek innovative solutions to combat climate change and reduce reliance on fossil fuels, breakthroughs like this highlight the critical role of scientific research and technological innovation. The work of Professor Yu Huang and her team at UCLA exemplifies the potential for academic research to drive meaningful progress in renewable energy, paving the way for a cleaner, more sustainable future.
Citation