Hydrogen from Water
Producing hydrogen from water may be achieved in several different ways such as electrolysis, direct solar, thermo-nuclear high temperature cracking, using catalysts, using biomaterials or using some form of chemicals to split water (H2O) into H2 (hydrogen) and O (oxygen).
 Hydrogen from Water |
AdvantagesAs you may already know approximately 70-percent of the Earth’s surface is covered with water. This makes water an attractive feedstock since it can be recycled back into nature indefinitely. When hydrogen is “burned” or oxidized in a fuel cell or internal combustion engine, it bonds with oxygen in the air, forms steam and drifts off into the atmosphere to become clouds. The clouds rain down water when can be used again to create hydrogen and the cycle continues. |
Disadvantages
One of the disadvantages of creating hydrogen from water is how much energy is used in the process and whether the process is clean beginning to end. For instance, simple brute force electrolysis of water takes a lot of energy and that energy will most likely for now be coal-powered.
The Right Way
The right way to make hydrogen from water is doing so in a renewable fashion. Wind, solar, geothermal and hydro-electric power can all be used in a renewable fashion to create hydrogen from water. Nuclear high-thermal cracking of water is considered by some people to also be renewable.
Electrolysis is perhaps the most sought after way to create hydrogen from water. However, the most popular way in general right now to create hydrogen is by high temperature steam reforming of natural gas, but this is for another discussion.
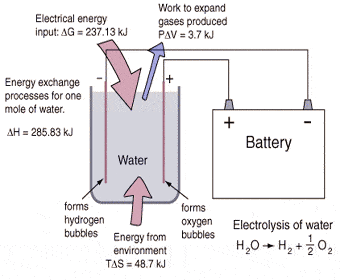
Like I’ve stated before, brute force electrolysis involves simply running an electrical current through distilled water splitting the H2O into H2 and O and then separating these two gases. In order to create hydrogen fuel for cars, capturing and compressing the H2 is very important.
Because the chemical bonds between the hydrogen atoms and oxygen atoms are so strong, scientists and researchers usually use some form of catalyst to loosen those bonds. PEM fuel cells are used to power most hydrogen cars. Hydrogen from a pressurized tank combines with oxygen from the air, which is forced into the fuel cell. This creates electricity as the hydrogen ions and oxygen ions travel around separate parts of the fuel cell and then merge on the backend to create steam and heat.
But, what a lot of people don’t realize is that fuel cells can be run in reverse. At the backend of the fuel cell water is input and the platinum inside the PEM acts as a metal catalyst so that not as much electricity needs to be input the split the water in to H2 and O.
HHO, HHO, HHO!
Some vehicle owners use hydrogen generators for cars to create HHO gas or H2 and O by splitting water onboard their vehicles (hydrogen on demand). They usually use a chemical catalyst such as KOH (potassium hydroxide) or NaOH (Sodium hydroxide). This means that not as much electricity from the battery or alternative need to be used in these hydrogen generators as would be the case if a person used distilled water and no additives.
Producing hydrogen from water can also be accomplished renewably, as already mentioned, and usually a catalyst is used in this process so that not as much electricity is needed in the process. There are also some other methods to create hydrogen from water that don’t involve electricity.
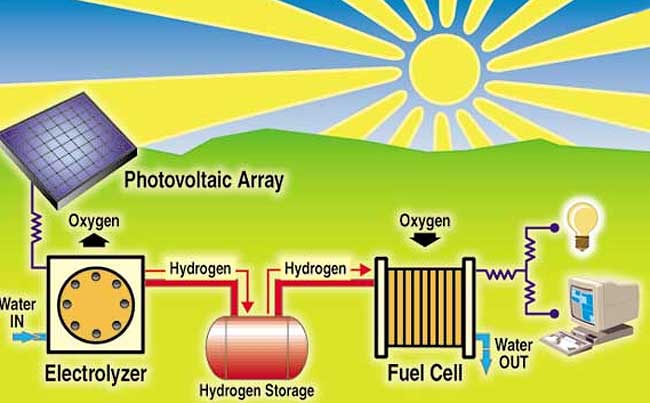
Scientists and researchers have been working at methods of direct solar conversion of water to hydrogen and oxygen that is couple with thermal cracking, biological cracking or chemical cracking of water. Some scientists aim to replicate photosynthesis where plants naturally take in water and sunlight and produce hydrogen and give off oxygen.
Solar to Hydrogen
Another method of creating hydrogen from water involves using both metal catalysts and chemical catalysts. One experiment (which can be quite dangerous so don’t try this at home) involves putting water inside and aluminum can and then adding drain cleaner. The metal and chemical reaction splits the water into hydrogen and oxygen. Some experimenters will capture the results with a balloon which is hot to the touch.
Another method of making hydrogen from water involves using particular biological microbes and a particular feedstock. Some water treatment plants have been experimenting in finding the right microbes to digest the water and waste and give off hydrogen in the process.
Thermo-nuclear high temperature cracking of water to make hydrogen is another method of H2 production that scientists are experimenting with for large scale needs such as powering hydrogen cars. Nuclear power plants use large quantities of water anyway to cool the core. This gives off steam.
Some researchers say why not use some of the electricity from the power plant combined with the high temperature steam and crack this steam into H2 and O using electrolysis? There has been some success in this area and if we are going to use nuclear energy why just throw away the waste steam instead of using it to create hydrogen? Of course if you are anti-nuclear in your beliefs and prefer solar and wind energy to carry us forward renewably then this will not be such a good idea.
These are just some of the ways of producing hydrogen from water. In order for zero emissions fuel cell cars to operate we need to find ways to create hydrogen fuel in mass quantities, economically, and using as little energy as possible in the production process. This is the only way H2 will ever compete with gasoline.
Written by Hydro Kevin Kantola